Mirimus, Inc. Announces Publication of Scientific Paper Highlighting Pooled Saliva-Based Testing as an Effective, Cost-Efficient Approach to COVID-19 Surveillance Testing for Schools
NEW YORK, Feb. 22, 2021 /PRNewswire/ — Mirimus, Inc., a leader in conducting high-volume, high-quality PCR testing, today announced the publication of a scientific paper, titled, “Pooled Surveillance Testing Program for Asymptomatic SARS-CoV-2 Infections in K-12 Schools and Universities,” available for preprint on MedRxiv as it undergoes scientific peer review for potential publication. The paper highlights the ability of Mirimus’ pioneering SalivaClear pooled saliva-based COVID-19 testing platform to provide schools and universities with an effective and cost-efficient approach to weekly COVID-19 surveillance testing.
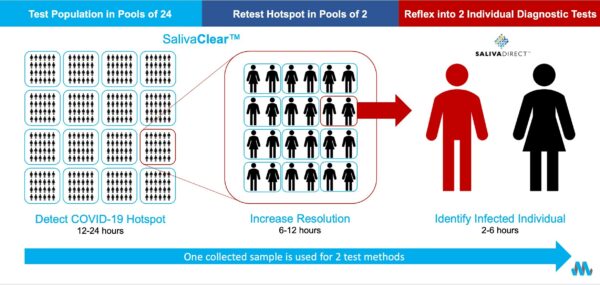
Developed by Mirimus as an alternative to individual PCR testing, the SalivaClear platform consists of three key elements – saliva-based sampling, pooled testing and gold-standard PCR molecular diagnostics – that, when combined, enable frequent, high-quality, high-throughput, low-cost detection of SARS-CoV-2, the virus that causes COVID-19. Rather than test individual students, faculty and staff separately, saliva samples from up to 24 individuals are combined, or pooled and then analyzed in a single test at the Mirimus laboratory, to save time and cut costs.
The study, coauthored by Prem Premsrirut, M.D., Ph.D., Co-founder and CEO of Mirimus, and researchers from institutions including SUNY Downstate Health University, Georgetown University, the Minnesota Timberwolves, the NBA Sports Science Committee, and The Yale School of Public Health determined that the pooling of saliva samples substantially reduced the cost associated with PCR testing and allowed schools to rapidly assess transmission and make data-driven decisions to adjust prevention protocols. Additionally, the research determined that SalivaClear provides a similar sensitivity to the molecular assay of individual samples, in terms of both qualitative (100% agreement of results on both pooled and individual samples) and quantitative (comparable cycle threshold values between pooled and individual samples) measures.
“The data compiled in our study leaves little doubt that saliva-based pooled testing should be a fundamental component to the reopening of schools given its ability to efficiently, accurately and cost-effectively detect COVID-19,” stated Dr. Premsrirut. “Based on our research, schools that performed regular surveillance testing with SalivaClear were able to quickly identify COVID-19 hotspots before they became outbreaks, providing critical, ‘real-time’ intelligence that is key to minimizing transmission among students and faculty. As a result, all schools utilizing the SalivaClear testing platform in this study were able to remain open to in-person learning.”
Over a 20-week period from August 2020 to January 2021, Mirimus collected 249,531 individual saliva samples from student, faculty and staff at 93 K-12 schools and then condensed those samples into 10,057 pools. An additional 15,802 saliva samples were collected from students, faculty and staff at 18 universities and colleges and condensed into 715 pools. These pools were tested at the Mirimus laboratories using the SalivaCleaar technology platform. Results from the pool testing were available and reported to the schools within 24 hours of sample collections, on average, and individual results were reported within 36 to 48 hours of sample collection.
A total of 863 positive individual samples (0.2% individual positivity rate) were detected using this method. In line with overall COVID-19 positivity trends, observable peaks were seen on days following long weekends and holidays. The highest number of positive pools was observed five days after New Year’s Eve (January 5, 2021; 39 positive pools, 230 were positive individuals). This was a significant increase in the number of positive samples (p=0.045) compared to any other testing day. The second highest number of positive pools was recorded on November 11 (29 positive pools; 32 positive individuals) and the second highest number of positive individual samples was observed on November 13 (28 positive pools; 50 positive individuals), 11 to 13 days after Halloween. This was also a significant increase when compared to the number of positive samples from prior dates (p=0.0048).
Dr. Premsrirut continued, “The positivity rates we measured provide two key insights. First, gathering in groups, such as a holiday celebration, significantly increases the transmission of COVID-19. And second, while in-school transmission of COVID-19 appears to be relatively low, students and faculty are just as susceptible to COVID-19 infection as any population group, which means schools must continue to be extremely vigilant with mitigation protocols, including regular testing, in order to minimize exposure.”
The research also evaluated the cost of the initial pooled testing, which was approximately $13 to $15 per individual for all participating schools. Reflex testing, which included the overall pooled to individual test, averaged $16 to $17 per person. This cost included all testing supplies, logistics, and personnel as well as diagnostic reporting, and allowed for over a dozen districts to participate in the study.
Dr. Premsrirut concluded, “In order to provide enough testing capacity to safely open schools and provide testing for at-risk communities, pooled concepts, such as SalivaClear, must be further evaluated and considered. Importantly, lower pricing and pooled approaches allow for a ‘multiplier effect’ that can provide significant economies of scale, potentially decreasing the costs even further at larger scale.”
More information on this study is available at https://www.medrxiv.org/content/10.1101/2021.02.09.21251464v1 . Mirimus has submitted the paper for potential publication in a peer-reviewed journal at a future date.
About SalivaClear
SalivaClear, developed by Mirimus, Inc., is a pioneering COVID-19 testing platform that combines saliva-based sampling, pooled testing and PCR diagnostics to enable high-quality, high-throughput, low-cost detection of SARS-CoV-2. SalivaClear is designed to accomplish what other COVID-19 testing approaches have thus far been unable to achieve – providing a SARS-CoV-2 diagnostic system that can be easily and repeatedly utilized by myriad organizations, from schools to businesses to government organizations, to test (and retest) groups of individuals in order to quickly isolate COVID-19 hotspots before they can become outbreaks. For more information, please visit www.salivaclear.com.
About Mirimus, Inc.
Since its founding 10 years ago, Mirimus, Inc. has established itself as a leader in conducting high-volume, complex and highly effective PCR testing. This unique skillset led Mirimus to develop SalivaClear, a COVID-19 testing platform that combines saliva-based sampling, pooled testing and PCR diagnostics to improve the scalability, efficiency, cost-effectiveness and accuracy of COVID-19 testing. For more information, please visit www.mirimus.com and www.salivaclear.com.
Media Contacts:
Tiberend Strategic Advisors, Inc.
Johanna Bennett & Ingrid Mezo
jbennett@tiberend.com / 1-212-375-2686
imezo@tiberend.com / 1-646-604-5150
SOURCE Mirimus, Inc.
Related Links
http://www.salivaclear.com

